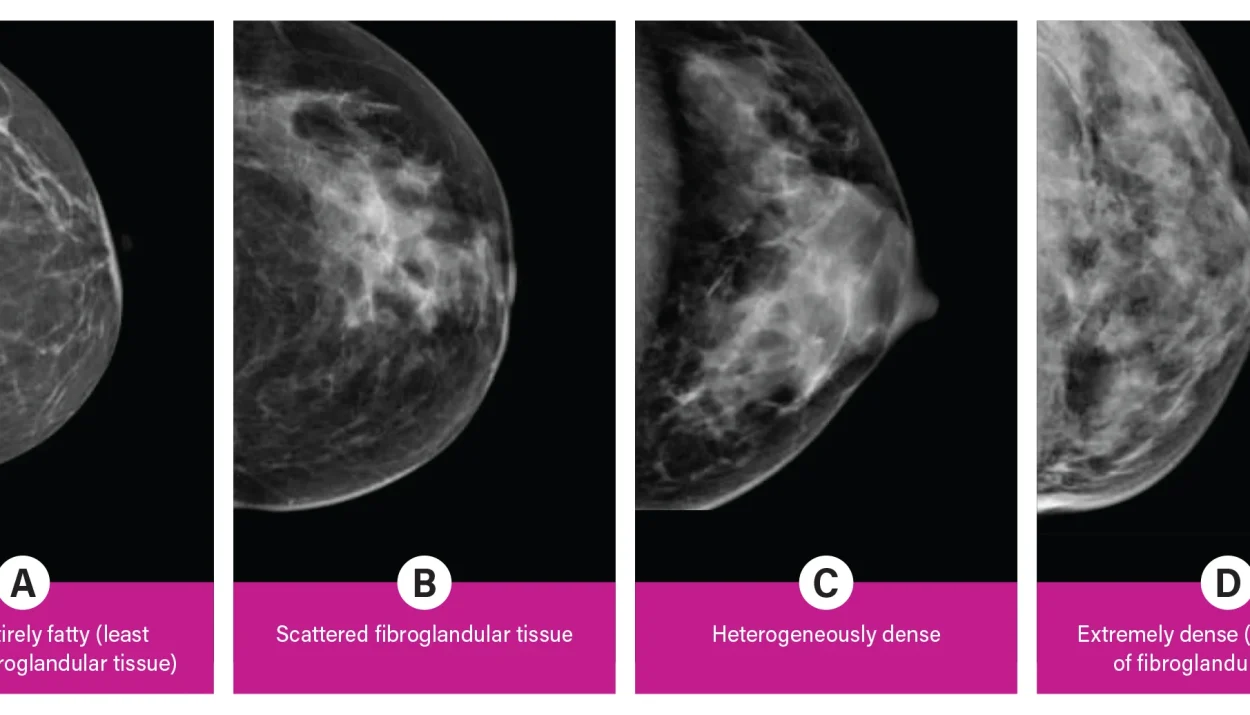
With the introduction of a new FDA rule, women aged 40 and above are now receiving notifications about their breast density along with the results of their routine mammograms. But not everyone agrees on what this means or how to act on this information.
For many women, like Shamma Mullen from Massachusetts, getting a letter about dense breasts can be both confusing and alarming. “I had never even heard of dense breasts before,” Mullen recalls, “and suddenly, I had questions I didn’t know I should be asking.” Like Mullen, countless women across the U.S. are facing this issue due to new notifications that explain whether their breast tissue is dense or not.
What Does Having Dense Breasts Mean?
Breast density refers to the proportion of milk glands and connective tissue compared to fatty tissue. Nearly half of women over the age of 40 have dense breasts, which can make it harder for mammograms to detect cancer. Additionally, women with dense breast tissue are at a slightly higher risk of developing breast cancer.
However, receiving this notification can often raise more questions than answers. Many women may not know what steps to take next, and health professionals themselves are divided on the best course of action. While some doctors recommend additional tests like ultrasounds or MRIs, others suggest sticking to regular mammograms.
Navigating Uncertainty: What’s Next?
The FDA hopes that these notifications will empower women to have more informed conversations with their doctors. Yet, the lack of a standardized approach to managing dense breasts has left many women feeling uncertain. Depending on where they live and who their doctor is, they might receive different advice on whether or not to pursue additional screenings.
While some health experts, such as Dr. Mark Pearlman from the University of Michigan Medical School, stress that “one-size-fits-all” policies don’t work for women with dense breasts, others are cautious about the risks of additional imaging. Dr. Nancy Keating of Harvard Medical School points out that while extra tests may detect more cancers, they don’t always save lives. In some cases, additional screenings can lead to false alarms or overdiagnosis, leading to unnecessary treatments.
Weighing the Benefits and Risks
Extra imaging tests, such as 3D mammograms or ultrasounds, come with their own set of risks. These tests can sometimes flag benign tissue as suspicious, leading to invasive biopsies or unnecessary follow-up tests. On the flip side, not every cancer found through additional testing needs immediate treatment, as some tumors grow so slowly they may never pose a serious health threat.
This nuanced issue has left many women, like Crissy Matos from Pennsylvania, questioning the consistency of their results. Matos had a standard mammogram at age 40 that showed no dense tissue, only to receive a dense breast notification two years later. For many, these mixed signals are confusing, making it hard to know how to respond.
Empowering Conversations
At its core, the FDA’s goal is to ensure that women have the information they need to make the best decisions for their health. However, experts caution that these notifications should not push women toward unnecessary tests without clear guidance.
As with any health issue, it’s essential for women to consult with their doctors and weigh the benefits and risks of additional screenings. While the notifications can spark important conversations, the best path forward will differ for every individual.
Ultimately, these breast density notifications aim to keep women informed. As Dr. Hilary Marston, the FDA’s chief medical officer, puts it, “We want women to have the knowledge to make informed choices for their health.” Yet, the complexity of breast density means that, for now, there is no definitive answer — only conversations that can guide each woman toward the right choice for her.